Passage
Solid-phase peptide synthesis is normally carried out on a resin bathed multiple times in dimethylformamide (DMF) -a toxic, polar, aprotic solvent-which poses an environmental problem because a large amount of toxic solvent waste is created. Cyclic carbonates such as propylene carbonate (PC) and ethylene carbonate (EC) are nontoxic, polar, aprotic solvents that could be used as an alternative to DMF.Compounds 1 and 2 are modified L-amino acids. They were coupled to form Compound 3, a dipeptide, which was then deprotected to yield Compound 4 (Figure 1) .
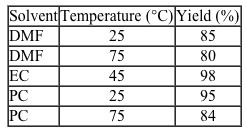
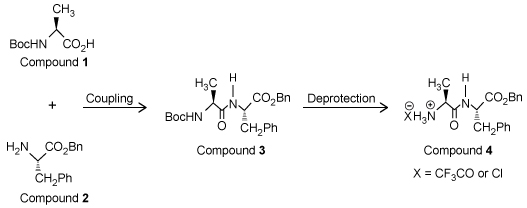 Figure 1 Synthesis of Compound 4To determine the effect that solvent type and temperature have on the synthesis of this peptide, the nontoxic solvents PC and EC as well as the toxic solvent DMF were each used in separate syntheses of Compound 4 at different temperatures. The reaction solvent was also used to rinse the resin. Table 1 shows the results of these syntheses.Table 1 Results from the Synthesis of Compound 4
Figure 1 Synthesis of Compound 4To determine the effect that solvent type and temperature have on the synthesis of this peptide, the nontoxic solvents PC and EC as well as the toxic solvent DMF were each used in separate syntheses of Compound 4 at different temperatures. The reaction solvent was also used to rinse the resin. Table 1 shows the results of these syntheses.Table 1 Results from the Synthesis of Compound 4
 The successful synthesis of Compound 4 using nontoxic solvents encouraged researchers to pursue the synthesis of a larger, biologically active peptide. Bradykinin, a vasodilator containing nine amino acids (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg) , was synthesized using the same solid-state peptide synthesis protocol used to synthesize Compound 4. Separate syntheses were done using either DMF or PC, and the purity of bradykinin from each synthesis was analyzed by high-performance liquid chromatography (HPLC) (Figure 2) .In each chromatogram, peak 1 has the same retention time as commercially obtained bradykinin, and the other peaks correspond to impurities. In chromatogram A, the impurity has a similar retention time to the product peak whereas the impurity peaks in chromatogram B have distinct retention times compared to the product peak.
The successful synthesis of Compound 4 using nontoxic solvents encouraged researchers to pursue the synthesis of a larger, biologically active peptide. Bradykinin, a vasodilator containing nine amino acids (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg) , was synthesized using the same solid-state peptide synthesis protocol used to synthesize Compound 4. Separate syntheses were done using either DMF or PC, and the purity of bradykinin from each synthesis was analyzed by high-performance liquid chromatography (HPLC) (Figure 2) .In each chromatogram, peak 1 has the same retention time as commercially obtained bradykinin, and the other peaks correspond to impurities. In chromatogram A, the impurity has a similar retention time to the product peak whereas the impurity peaks in chromatogram B have distinct retention times compared to the product peak.
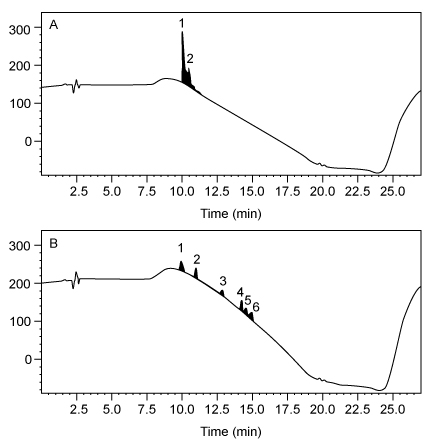 Figure 2 HPLC of synthetic bradykinin: (A) synthesis in DMF; (B) synthesis in PC
Figure 2 HPLC of synthetic bradykinin: (A) synthesis in DMF; (B) synthesis in PC
Adapted from S. B. Lawrenson et al., "The greening of peptide synthesis." Green Chem. ©2017 The Royal Society of Chemistry.
-What is the byproduct of the formation of Compound 3 described in the passage?
A) H2O; peptide bond formation is a dehydration reaction.
B) H2O; peptide bond formation is a hydrolysis reaction.
C) CO2; peptide bond formation is a decarboxylation reaction.
D) CO2; peptide bond formation is a coupling reaction.
Correct Answer:
Verified
Q120: Phospholipids contain all of the following structural
Q121: Passage
Fatty acids (FAs) are lipids that play
Q122: Passage
Solid-phase peptide synthesis is normally carried out
Q123: Passage
Solid-phase peptide synthesis is normally carried out
Q124: The experimental data obtained when a chiral
Q126: Passage
Fatty acids (FAs) are lipids that play
Q127: Passage
Fatty acids (FAs) are lipids that play
Q128: Passage
Chloramphenicol (Compound 1) is an antibiotic that
Q129: Passage
Chloramphenicol (Compound 1) is an antibiotic that
Q130: Passage
Malaria is caused by the parasite Plasmodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents