At 100 C, the following two reactions have the denoted equilibrium constants. 
What is the value of the equilibrium constant for the reaction?
2NOBr(g) + Cl2(g) 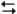 2NO(g) + 2BrCl(g)
2NO(g) + 2BrCl(g)
A) 180
B) 0.055
C) 3.125
D) .320
E) None of the above
Correct Answer:
Verified
Q1: Consider the following reaction in aqueous solution:
2A
Q2: At 25°C, Kp=2.86´1024 for the reaction:
2SO2(g) +
Q3: The following question(s) pertain to the
Q4: pertain to the equilibrium 2NO(g) +
Q5: pertain to the equilibrium 2NO(g) +
Q7: pertain to the reaction H2(g) + I2(g)
Q8: pertain to the reaction H2(g) + I2(g)
Q9: pertain to the reaction H2(g) + I2(g)
Q10: pertain to the reaction N2(g) + 3H2(g)
Q11: pertain to the reaction N2(g) + 3H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents