pertain to the reaction H2(g) + I2(g) 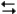 2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
2HI(g) at 150°C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm.
-Refer to Exhibit 14-2. Based on the equilibrium constant and the value of Q, will more reactants or products be formed as the system approaches equilibrium at this temperature?
A) More products will be formed
B) More reactants will be formed
C) Not enough data to answer the question
D) None of the above
Correct Answer:
Verified
Q3: The following question(s) pertain to the
Q4: pertain to the equilibrium 2NO(g) +
Q5: pertain to the equilibrium 2NO(g) +
Q6: At 100 C, the following two reactions
Q7: pertain to the reaction H2(g) + I2(g)
Q9: pertain to the reaction H2(g) + I2(g)
Q10: pertain to the reaction N2(g) + 3H2(g)
Q11: pertain to the reaction N2(g) + 3H2(g)
Q12: pertain to the reaction N2(g) + 3H2(g)
Q13: At room temperature cyclohexane exists almost exclusively
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents