pertain to the reaction N2(g) + 3H2(g) 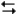 2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
2NH3(g) at 200°C with the reaction vessel being charged in such a way that the initial partial pressures of N2, H2, and NH3 are 0.200, 0.300, and 0.600 atm respectively.
-Refer to Exhibit 14-3. What is the value of the equilibrium constant Kp at this temperature?
A) 0.652
B) 2.78´10-21
C) 3.59´1020
D) 1.92´10-14
E) None of the above
Correct Answer:
Verified
Q3: The following question(s) pertain to the
Q4: pertain to the equilibrium 2NO(g) +
Q5: pertain to the equilibrium 2NO(g) +
Q6: At 100 C, the following two reactions
Q7: pertain to the reaction H2(g) + I2(g)
Q8: pertain to the reaction H2(g) + I2(g)
Q9: pertain to the reaction H2(g) + I2(g)
Q11: pertain to the reaction N2(g) + 3H2(g)
Q12: pertain to the reaction N2(g) + 3H2(g)
Q13: At room temperature cyclohexane exists almost exclusively
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents