Which equilibrium constant represents a reaction that favors the reactants to the greatest extent?
A) 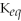
= 100
B) 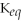
= 1.0 ×
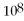
C) 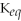
= 1.0 ×
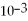
D) 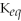
= 1.0 ×
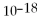
E) not enough information
Correct Answer:
Verified
Q75: For the reaction Q76: For the reaction 2 H2O (l)⇌ 2H2 Q77: Which equilibrium constant represents a reaction that Q77: When writing the expression for an equilibrium Q78: For the reaction LiOH (s)⇌ Li+ (aq)+ Q80: Identify the equation for which Q81: Consider the following endothermic equilibrium reaction: Q82: Which of the following equilibrium systems will Q83: For the reaction 2 Q84: For the reaction 2 Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()

Fe2O3 (s)+![]()
![]()

