Multiple Choice
Which of the following equilibrium systems will shift to the right (towards products) when pressure is increased.
A) 2 PbS (s) + 3O2 (g) ⇌ 2PbO (s) + 2 SO2 (g)
B) PCl5 (g) ⇌ PCl3 (g) + Cl2 (g)
C) H2 (g) + CO2 (g) ⇌ 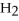
O (g) + CO (g)
D) all of these
E) none of these
Correct Answer:
Verified
Related Questions
Q77: Which equilibrium constant represents a reaction that
Q78: For the reaction LiOH (s)⇌ Li+ (aq)+
Q79: Which equilibrium constant represents a reaction that
Q80: Identify the equation for which 
Q81: Consider the following endothermic equilibrium reaction:
Fe2O3 (s)+
Q82: What happens to the equilibrium position of

