Multiple Choice
Consider the following endothermic equilibrium reaction:
Fe2O3 (s) + 3H2 (g) ⇌ 2Fe (s) + 3 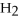
O (g)
Which of the following actions will result in a shift to the left (towards reactants) ?
A) increasing the temperature
B) increasing the pressure in the container
C) removing H2O (g)
D) all of these
E) none of these
Correct Answer:
Verified
Related Questions


