Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: 2NF3(g) 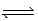 N2(g) + 3F2(g)
N2(g) + 3F2(g)
2) 50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0194 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L⋅atm/mol⋅K)
A) 2.68 × 10-6
B) 1.91 × 10-3
C) 1.79 × 10-3
D) 2.77 × 10-6
E) 4.43 × 10-7
Correct Answer:
Verified
Q17: Given the following chemical equilibrium COCl2(g)
Q18: When gaseous carbon monoxide and hydrogen are
Q19: Which of the following is the correct
Q20: What is the expression for Kc for
Q21: Exactly 1.0 mol N2O4 is placed in
Q23: The reaction quotient,Q,for a system is
Q24: An aqueous mixture of phenol and ammonia
Q25: The following reaction occurred when a 1.0-liter
Q26: A 10.0 g sample of solid NH4Cl
Q27: Consider the reaction A(aq) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents