The reaction quotient,Q,for a system is 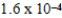 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 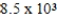 ,what will happen as the reaction mixture returns to equilibrium?
,what will happen as the reaction mixture returns to equilibrium?
A) The equilibrium constant will increase until it equals the reaction quotient.
B) There will be a net loss in both product(s) and reactant(s) .
C) There will be a net loss in product(s) .
D) There will be a net loss in reactant(s) .
E) The equilibrium constant will increase.
Correct Answer:
Verified
Q18: When gaseous carbon monoxide and hydrogen are
Q19: Which of the following is the correct
Q20: What is the expression for Kc for
Q21: Exactly 1.0 mol N2O4 is placed in
Q22: Nitrogen trifluoride decomposes at to form nitrogen
Q24: An aqueous mixture of phenol and ammonia
Q25: The following reaction occurred when a 1.0-liter
Q26: A 10.0 g sample of solid NH4Cl
Q27: Consider the reaction A(aq) Q28: Consider the reaction below. 2 HF(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents