Multiple Choice
Consider the reaction below. 2 HF(g) 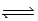 H2(g) + F2(g) (Kc = 1.00 × 10-2)
H2(g) + F2(g) (Kc = 1.00 × 10-2)
Given that 1.00 mol of HF(g) ,0.241 mol of H2(g) ,and 0.750 mol of F2(g) are mixed in a 5.00 L flask,determine the reaction quotient,Q.
A) Q = 0.0452
B) Q = 0.181
C) Q = 0.0362
D) Q = 1.99
E) None of these
Correct Answer:
Verified
Related Questions
Q23: The reaction quotient,Q,for a system is
Q24: An aqueous mixture of phenol and ammonia
Q25: The following reaction occurred when a 1.0-liter
Q26: A 10.0 g sample of solid NH4Cl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents