The thermochemical equation for the combustion of methanol is given below. CH3OH( 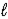 ) + 3/2 O2(g) → CO2(g) + 2 H 2O(g)
) + 3/2 O2(g) → CO2(g) + 2 H 2O(g)
ΔrH° = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g of CH3OH?
A) -171 kJ
B) -19.9 kJ
C) -2.38 × 103 kJ
D) -5.49 × 103 kJ
E) -1.76 × 106 kJ
Correct Answer:
Verified
Q18: Specific heat capacity is
A) the quantity of
Q19: A 170.0-g sample of metal at 83.00°C
Q20: Many homes are heated using natural gas.The
Q21: The heat of vaporization of benzene,C6H6,is 30.7
Q22: Given the thermochemical equation 4AlCl3(s)+ 3O2(g)→ 2Al2O3(s)+
Q24: Commercial cold packs consist of solid ammonium
Q25: The thermochemical equation for the combustion of
Q26: Calculate the energy in the form of
Q27: The following reaction of iron oxide with
Q28: 44.0 g of ice at -20.0 °C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents