A bomb calorimeter has a heat capacity of 2.47 kJ/K.When a 0.103-g sample of a certain hydrocarbon was burned in this calorimeter,the temperature increased by 2.14 K.Calculate the energy of combustion for 1 g of the hydrocarbon.
A) -5.29 J/g
B) 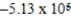 J/g
J/g
C) -0.120 J/g
D) 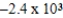 J/g
J/g
E) -0.540 J/g
Correct Answer:
Verified
Q50: In thermodynamics,a(n)_ is defined as the object,or
Q51: Using the following thermochemical data: 2Sm(s)+ 6HF(g)→
Q52: Acetylene,C2H2,is a gas used in welding.The molar
Q53: When 0.265 mol of a weak base
Q54: Determine the enthalpy change for the decomposition
Q56: Combustion of 2.14 g of liquid benzene
Q57: Which of the following chemical equations does
Q58: The heat required to convert a solid
Q59: Which of the following has zero standard
Q60: When 1 mole of Fe2O3(s)reacts with H2(g)to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents