Combustion of 2.14 g of liquid benzene (C6H6) causes a temperature rise of 16.2 °C in a constant-pressure calorimeter that has a heat capacity of 5.53 kJ/°C.What is ΔH for the following reaction? C6H6(l) + 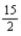 O2(g) → 6 CO2(g) + 3 H2O(
O2(g) → 6 CO2(g) + 3 H2O(  )
)
A) -89.5 kJ/mol-rxn
B) 41.8 kJ/mol-rxn
C) -41.8 kJ/mol-rxn
D) -3.27 × 103 kJ/mol-rxn
E) 89.5 kJ/mol-rxn
Correct Answer:
Verified
Q51: Using the following thermochemical data: 2Sm(s)+ 6HF(g)→
Q52: Acetylene,C2H2,is a gas used in welding.The molar
Q53: When 0.265 mol of a weak base
Q54: Determine the enthalpy change for the decomposition
Q55: A bomb calorimeter has a heat capacity
Q57: Which of the following chemical equations does
Q58: The heat required to convert a solid
Q59: Which of the following has zero standard
Q60: When 1 mole of Fe2O3(s)reacts with H2(g)to
Q61: Dry ice converts directly from a solid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents