Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride (M = 102.1 g/mol) . C7H6O3(s) + C4H6O3( 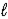 ) → C9H8O4(s) + C2H4O2(
) → C9H8O4(s) + C2H4O2( 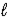 )
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol) is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%
Correct Answer:
Verified
Q33: Sulfur hexafluoride is produced by reacting elemental
Q34: The reaction of 0.779 g K with
Q35: Chlorine was passed over 1.06 g of
Q36: A 1.850 g mixture of SrCO3 and
Q37: Under certain conditions the reaction of ammonia
Q39: A certain compound has a molar mass
Q40: Complete combustion of a 0.70-mol sample of
Q41: What is the molarity of an NaI
Q42: What is the pH of 4.1 ×
Q43: A 1.078 g sample of a compound
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents