The temperature rises from 25.00 °C to 29.00 °C when 3.50 g of sucrose undergoes combustion in a bomb calorimeter. Calculate 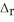 U for the combustion of sucrose in kJ
U for the combustion of sucrose in kJ 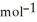 sucrose. The heat capacity of the calorimeter is 4.90 kJ
sucrose. The heat capacity of the calorimeter is 4.90 kJ 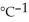 . The molar mass of sugar is 342.3 g
. The molar mass of sugar is 342.3 g 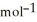 .
.
A) -1.92 × 103 kJ mol-1
B) 1.92 × 103 kJ mol-1
C) -1.23 × 103 kJ mol-1
D) 2.35 × 104 kJ mol-1
Correct Answer:
Verified
Q46: A 35.6 g sample of ethanol (C2H5OH)
Q47: A 6.55 g sample of aniline (C6H5NH2,
Q48: Which of the following statements is TRUE?
A)
Q49: Using the following equation for the combustion
Q50: Using the following thermochemical equation, determine the
Q52: Using the following equation for the combustion
Q53: Which of the following processes is endothermic?
A)
Q54: Given w = 0, an endothermic reaction
Q55: How much energy is evolved during the
Q56: Using the following equation for the combustion
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents