A 35.6 g sample of ethanol (C2H5OH) is burned in a bomb calorimeter according to the following reaction. If the temperature rose from 35.0 °C to 76.0°C and the heat capacity of the calorimeter is 23.3 kJ 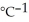 , what is the value of ΔU°? The molar mass of ethanol is 46.07 g
, what is the value of ΔU°? The molar mass of ethanol is 46.07 g 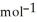 . C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g) ΔU° = ?
. C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g) ΔU° = ?
A) -1.24 × 103 kJ mol-1
B) +1.24 × 103 kJ mol-1
C) -8.09 × 103 kJ mol-1
D) -9.55 × 103 kJ mol-1
E) +9.55 × 103 kJ mol-1
Correct Answer:
Verified
Q41: Which of the following processes is exothermic?
A)
Q42: According to the following reaction, how much
Q43: How much energy is evolved during the
Q44: How much energy is required to decompose
Q45: Using the following thermochemical equation, determine the
Q47: A 6.55 g sample of aniline (C6H5NH2,
Q48: Which of the following statements is TRUE?
A)
Q49: Using the following equation for the combustion
Q50: Using the following thermochemical equation, determine the
Q51: The temperature rises from 25.00 °C to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents