How much energy is evolved during the reaction of 48.7 g of Al according to the reaction below? Assume that there is excess
Fe2O3. Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 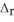 H° = -852 kJ
H° = -852 kJ
A) 415 kJ
B) 207 kJ
C) 241 kJ
D) 130 kJ
E) 769 kJ
Correct Answer:
Verified
Q38: An 8.21 g sample of glycerol (C3H8O3,
Q39: Identify what a bomb calorimeter measures.
A) measures
Q40: Calculate the internal energy change, ΔrU, for
Q41: Which of the following processes is exothermic?
A)
Q42: According to the following reaction, how much
Q44: How much energy is required to decompose
Q45: Using the following thermochemical equation, determine the
Q46: A 35.6 g sample of ethanol (C2H5OH)
Q47: A 6.55 g sample of aniline (C6H5NH2,
Q48: Which of the following statements is TRUE?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents