According to the following reaction, how much energy is required to decompose 55.0 kg of Fe3O4? The molar mass of Fe3O4 is 231.55 g 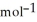 . Fe3O4(s) → 3Fe(s) + 2O2(g)
. Fe3O4(s) → 3Fe(s) + 2O2(g)  H° = +1118 kJ
H° = +1118 kJ
A) 1.10 × 106 kJ
B) 2.38 × 102 kJ
C) 2.66 × 105 kJ
D) 1.12 × 103 kJ
E) 3.44 × 104 kJ
Correct Answer:
Verified
Q37: Calculate the change in internal energy (ΔU)
Q38: An 8.21 g sample of glycerol (C3H8O3,
Q39: Identify what a bomb calorimeter measures.
A) measures
Q40: Calculate the internal energy change, ΔrU, for
Q41: Which of the following processes is exothermic?
A)
Q43: How much energy is evolved during the
Q44: How much energy is required to decompose
Q45: Using the following thermochemical equation, determine the
Q46: A 35.6 g sample of ethanol (C2H5OH)
Q47: A 6.55 g sample of aniline (C6H5NH2,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents