Using the following equation for the combustion of octane, calculate the amount of moles of oxygen that reacts with 100.0 g of octane. The molar mass of octane is 114.33 g 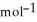 . The molar mass of carbon dioxide is 44.0095 g
. The molar mass of carbon dioxide is 44.0095 g 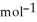 . 2C8H18 + 25O2 → 16CO2 + 18H2O
. 2C8H18 + 25O2 → 16CO2 + 18H2O 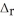 H° = -11018 kJ
H° = -11018 kJ
A) 18.18 moles
B) 6.997 moles
C) 14.00 moles
D) 8.000 moles
E) 10.93 moles
Correct Answer:
Verified
Q47: A 6.55 g sample of aniline (C6H5NH2,
Q48: Which of the following statements is TRUE?
A)
Q49: Using the following equation for the combustion
Q50: Using the following thermochemical equation, determine the
Q51: The temperature rises from 25.00 °C to
Q53: Which of the following processes is endothermic?
A)
Q54: Given w = 0, an endothermic reaction
Q55: How much energy is evolved during the
Q56: Using the following equation for the combustion
Q57: According to the following reaction, how much
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents