According to the following reaction, how much energy is evolved during the reaction of 32.5 g B2H6 and 72.5 g Cl2? The molar mass of B2H6 is 27.67 g 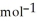 . B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g)
. B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g) 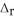 H° = -1396 kJ
H° = -1396 kJ
A) 1640 kJ
B) 238 kJ
C) 1430 kJ
D) 3070 kJ
E) 429 kJ
Correct Answer:
Verified
Q52: Using the following equation for the combustion
Q53: Which of the following processes is endothermic?
A)
Q54: Given w = 0, an endothermic reaction
Q55: How much energy is evolved during the
Q56: Using the following equation for the combustion
Q58: Which of the following processes is endothermic?
A)
Q59: A 12.8 g sample of ethanol (C2H5OH)
Q60: A bomb calorimeter with a heat capacity
Q61: Which of the following statements is TRUE?
A)
Q62: Calculate the heat transfer, in J, when
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents