A gas vessel is attached to an open- end manometer containing a nonvolatile liquid of density 0.791 g/mL as shown below.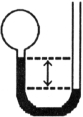
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
A) 0.993
B) 1.03
C) 0.987
D) 0.960
E) 0.990
Correct Answer:
Verified
Q107: The volume occupied by 1.5 mol of
Q108: A flask contains a mixture of He
Q109: A sample of gas (24.2 g) initially
Q110: A gas at a pressure of 10.0
Q111: The pressure exerted by 1.3 mol of
Q113: The reaction of 50 mL of N2
Q114: In a Torricelli barometer, a pressure of
Q115: SO2 (5.00 g) and CO2 (5.00 g)
Q116: Sodium hydride reacts with excess water to
Q117: CO (5.00 g) and CO2 (5.00 g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents