When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction, 98.8 kJ of energy is absorbed.
Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 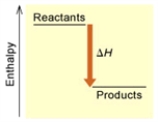 (A)
(A) 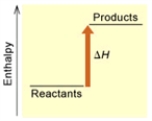
(B)
Is the reaction endothermic or exothermic, and which of the enthalpy diagrams above
Represents this reaction?
A) Endothermic; A
B) Endothermic; B
C) Exothermic; A
D) Exothermic; B
E) None of these
Correct Answer:
Verified
Q42: What is the overall chemical equation that
Q50: In thermodynamics,a(n)_ is defined as the object,or
Q58: The heat required to convert a solid
Q59: Combustion of 2.14 g of liquid
Q61: Dry ice converts directly from a solid
Q62: Internal energy and enthalpy are state functions.What
Q63: According to the reaction given below,
Q63: _ is used to measure the energy
Q65: Why are you at greater risk from
Q67: The standard molar enthalpy of formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents