Propane is a fuel commonly used in barbeques and to heat homes.It has the structure shown below.Does propane have a permanent dipole? 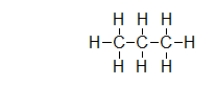
A) Yes.The carbon is partially negative, and the hydrogen is partially positive.
B) Yes.The carbon is partially positive, and the hydrogen is partially negative.
C) Yes.One side of the molecule is always partially positive, and the other side is partially negative.
D) No.Propane has a temporary dipole.
E) No.Propane never displays any dipole at all.
Correct Answer:
Verified
Q2: An atom in a molecule has one
Q3: The electronegativity difference between C and O
Q4: What is the electron geometry of the
Q5: The electronegativity difference between K and Cl
Q6: What is the electron geometry of the
Q7: What is the molecular geometry of the
Q8: Which figure BEST illustrates how two molecules
Q9: Electrostatic interactions between positive and negative ions
Q10: An atom, X, has a tetrahedral electron
Q11: Which of the following covalent compounds is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents