An atom has atomic number 92 and mass number 238. Which symbol is an ISOTOPE of this atom?
A) 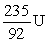
B) 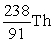
C) 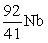
D) 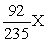
Correct Answer:
Verified
Q20: Which of these symbols represents an atom
Q21: Which symbol BEST represents a potassium cation?
A)
Q22: Which symbol represents an atom with 8
Q24: A certain element is composed of two
Q25: Which scientist proposed that electrons orbit the
Q26: Which symbol BEST represents a lithium cation?
A)
Q27: Which symbol represents an atom with 9
Q28: A certain element is composed of two
Q29: An atom that loses an electron is:
A)
Q30: An atom that gains an electron is:
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents