Hydrogen sulfide can be formed in the following reaction: 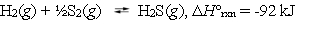 The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
Correct Answer:
Verified
Q69: Ethane can be formed by reacting acetylene
Q70: Hydrogen bromide will dissociate into hydrogen
Q71: Stearic acid, nature's most common fatty
Q72: The reaction system Q73: Methanol can be synthesized by combining carbon Q75: The following reaction is at equilibrium in Q76: A container was charged with hydrogen, Q77: The following reaction is at equilibrium in Q78: The following reaction is at equilibrium at Q79: The reaction system ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents