Multiple Choice
Stearic acid, nature's most common fatty acid, dimerizes when dissolved in hexane: 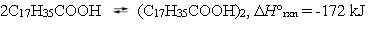 The equilibrium constant for this reaction at 28 C is 2900. Estimate the equilibrium constant at 38 C.
The equilibrium constant for this reaction at 28 C is 2900. Estimate the equilibrium constant at 38 C.
A) 4.7 * 105
B) 2.6 * 104
C) 1.9 * 103
D) 3.2 * 102
E) 18
Correct Answer:
Verified
Related Questions
Q66: The reaction of nitric oxide to
Q67: At 450
Q68: Sodium hydrogen carbonate decomposes above 110
Q69: Ethane can be formed by reacting acetylene
Q70: Hydrogen bromide will dissociate into hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents