At 450 C, tert-butyl alcohol decomposes into water and isobutene. 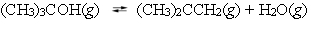 A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
A reaction vessel contains these compounds at equilibrium. What will happen if the volume of the container is reduced by 50% at constant temperature?
A) The forward reaction will proceed to reestablish equilibrium.
B) The reverse reaction will proceed to reestablish equilibrium.
C) No change occurs.
D) The equilibrium constant will increase.
E) The equilibrium constant will decrease.
Correct Answer:
Verified
Q62: Nitrogen dioxide can dissociate to nitric oxide
Q63: The following reaction is at equilibrium at
Q64: The reaction system Q65: Magnesium hydroxide is used in several Q66: The reaction of nitric oxide to Q68: Sodium hydrogen carbonate decomposes above 110 Q69: Ethane can be formed by reacting acetylene Q70: Hydrogen bromide will dissociate into hydrogen Q71: Stearic acid, nature's most common fatty Q72: The reaction system ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents