The reaction system 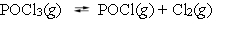 is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
A) POCl3 will be consumed as equilibrium is established.
B) POCl will be consumed as equilibrium is established.
C) Chlorine will be consumed as equilibrium is established.
D) The partial pressure of POCl will decrease while the partial pressure of Cl2 increases as equilibrium is established.
E) The volume will have to decrease before equilibrium can be reestablished.
Correct Answer:
Verified
Q59: At a certain temperature the reaction CO2(g)
Q60: Compounds A, B, and C react
Q61: Magnesium carbonate dissociates to magnesium oxide
Q62: Nitrogen dioxide can dissociate to nitric oxide
Q63: The following reaction is at equilibrium at
Q65: Magnesium hydroxide is used in several
Q66: The reaction of nitric oxide to
Q67: At 450
Q68: Sodium hydrogen carbonate decomposes above 110
Q69: Ethane can be formed by reacting acetylene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents