Nitrogen dioxide can dissociate to nitric oxide and oxygen. 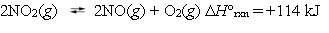 Under which reaction conditions would you expect to produce the largest amount of oxygen?
Under which reaction conditions would you expect to produce the largest amount of oxygen?
A) high temperature, high pressure
B) low temperature, high pressure
C) high temperature, low pressure
D) low temperature, low pressure
E) None of these choices is correct, unless a catalyst is present
Correct Answer:
Verified
Q57: At 25
Q58: Nitric oxide is formed in automobile
Q59: At a certain temperature the reaction CO2(g)
Q60: Compounds A, B, and C react
Q61: Magnesium carbonate dissociates to magnesium oxide
Q63: The following reaction is at equilibrium at
Q64: The reaction system Q65: Magnesium hydroxide is used in several Q66: The reaction of nitric oxide to Q67: At 450 ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents