The reaction of nitric oxide to form dinitrogen oxide and nitrogen dioxide is exothermic. 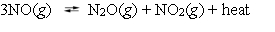 What effect will be seen if the temperature of the system at equilibrium is raised by 25 C?
What effect will be seen if the temperature of the system at equilibrium is raised by 25 C?
A) The partial pressure of NO will increase.
B) The partial pressure of NO will decrease.
C) The partial pressure of NO2 will increase.
D) The partial pressures of NO and N2O will increase.
E) All three partial pressures will increase.
Correct Answer:
Verified
Q61: Magnesium carbonate dissociates to magnesium oxide
Q62: Nitrogen dioxide can dissociate to nitric oxide
Q63: The following reaction is at equilibrium at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents