The reaction system 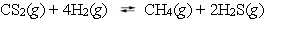 is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
A) As equilibrium is reestablished, the partial pressure of carbon disulfide increases.
B) As equilibrium is reestablished, the partial pressure of methane, CH4, decreases.
C) As equilibrium is reestablished, the partial pressure of hydrogen decreases.
D) As equilibrium is reestablished, the partial pressure of hydrogen sulfide decreases.
E) As equilibrium is reestablished, all the partial pressures will decrease.
Correct Answer:
Verified
Q67: At 450
Q68: Sodium hydrogen carbonate decomposes above 110
Q69: Ethane can be formed by reacting acetylene
Q70: Hydrogen bromide will dissociate into hydrogen
Q71: Stearic acid, nature's most common fatty
Q73: Methanol can be synthesized by combining carbon
Q74: Hydrogen sulfide can be formed in the
Q75: The following reaction is at equilibrium in
Q76: A container was charged with hydrogen,
Q77: The following reaction is at equilibrium in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents