Ammonium nitrate is an ingredient in cold packs used by sports trainers for injured athletes.Calculate the change in temperature when 42 g of ammonium nitrate (NH4NO3, 80.1 g/mol) dissolves in 250 g water.Assume the specific heat of the solution is 4.18 J/(g . C).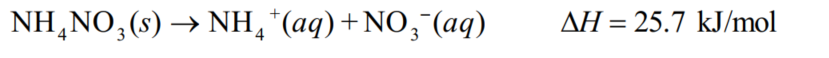
Correct Answer:
Verified
Q183: In an experiment, 100.0 g of
Q184: The molar heat capacities of zinc,
Q185: In an experiment, a glass marble
Q186: You wish to devise a way
Q187: Suppose 125 grams of aluminum at
Q189: Suppose 250 g of water at
Q190: In an experiment, 9.80 g of
Q191: The destruction of the ozone layer
Q192: Calculate the enthalpy of solution for
Q193: In an experiment, 30.00 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents