Calculate the Enthalpy of Solution for Calcium Chloride (110 CAssume the Specific Heat of the Solution Is 4
Calculate the enthalpy of solution for calcium chloride (110.98 g/mol) using the following data: When 37.73 g of CaCl2 is dissolved in 240.00 g of water, the temperature of the solution increases by 24.25 C.Assume the specific heat of the solution is 4.18 J/(g . C).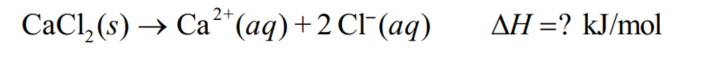
Correct Answer:
Verified
Q187: Suppose 125 grams of aluminum at
Q188: Ammonium nitrate is an ingredient in
Q189: Suppose 250 g of water at
Q190: In an experiment, 9.80 g of
Q191: The destruction of the ozone layer
Q193: In an experiment, 30.00 g of
Q194: Benzoic acid is used to determine
Q195: Suppose 23.00 g ammonium chloride 53.491
Q196: The combustion of liquefied butane to
Q197: Suppose 3.03 kg of asphalt at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents