Suppose 23.00 g ammonium chloride 53.491 (g/mol) dissolves in water in a calorimeter to form 100.00 g of solution.The temperature decreases by 10.75 C.Calculate the H of solution of NH4Cl in kJ/mol.Assume the specific heats of the solution and of the empty calorimeter are (4.18 J/g . C) and 0.170 kJ/ C, respectively.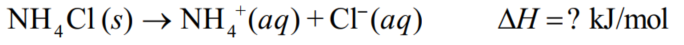
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q190: In an experiment, 9.80 g of
Q191: The destruction of the ozone layer
Q192: Calculate the enthalpy of solution for
Q193: In an experiment, 30.00 g of
Q194: Benzoic acid is used to determine
Q196: The combustion of liquefied butane to
Q197: Suppose 3.03 kg of asphalt at
Q198: To cool 250 mL of coffee
Q199: When magnesium reacts with hydrochloric acid,
Q200: The complete combustion of 2.500 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents