When magnesium reacts with hydrochloric acid, aqueous magnesium chloride and hydrogen gas are produced.Suppose 0.120 g Mg react with the HCl in a 75.000 g acidic solution contained in a calorimeter.The temperature increases by 6.03 C.Calculate the Hrxn of Mg with HCl in kJ/mol.Assume the specific heats of the solution and of the empty calorimeter are 4.18 J/g . C) and 64.4 J/ C, respectively.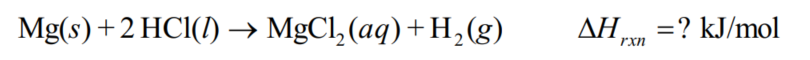
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q123: Define the terms enthalpy of solution, enthalpy
Q130: Motor fuels are mixtures of hydrocarbons, but
Q194: Benzoic acid is used to determine
Q195: Suppose 23.00 g ammonium chloride 53.491
Q196: The combustion of liquefied butane to
Q197: Suppose 3.03 kg of asphalt at
Q198: To cool 250 mL of coffee
Q200: The complete combustion of 2.500 g
Q203: When ammonium nitrate (NH4NO3)(s) is used in
Q204: Use the following data to calculate the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents