An ideal gas undergoes the process shown in the diagram. In this figure, , and . How much work is done by the system in this process?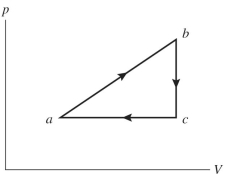
A)
B)
C)
D)
E)
Correct Answer:
Verified
Q20: A 10-L flask and a 1-L
Q21: The figure shows a
Q22: A gas expands from an initial
Q23: An ideal gas undergoes the process
Q24: A certain automobile engine takes in 4.00
Q26: The figure shows a
Q27: During each cycle, a heat engine takes
Q28: A 40.0-L container is divided into
Q29: A heat engine absorbs 64 kcal of
Q30: The figure shows a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents