True/False
The substance in the figure with the most atoms per mole is NaCl. 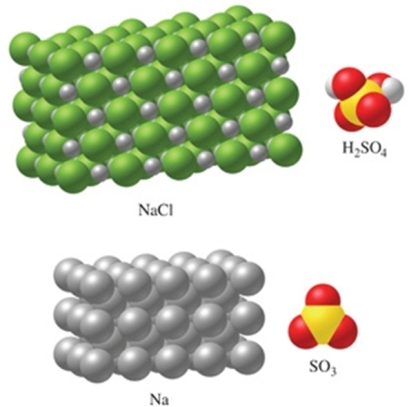
Correct Answer:
Verified
Related Questions
Q101: If 50.0 mL of 0.235 M NaCl
Q103: A formula written with the simplest ratio
Q103: The solute in the solution shown in
Q105: If 100.0 mL of 0.426 M NaOH
Q109: One mole of methane (CH4)and one mole
Q110: Of the substances shown in the figure,
Q111: Image B in the figure shows a
Q117: The process of diluting a solution in
Q123: Molarity is defined as the moles of
Q129: To obtain 0.50 moles of potassium nitrate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents