A carbene in the singlet state is typically portrayed as sp2 hybridized, with a vacant p -orbital and a pair of nonbonding electrons residing in one sp2 hybrid orbital. Using molecular orbital theory, describe how a neighboring 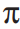 -bond can act as a nucleophile with the carbene in the formation of a cyclopropyl ring.
-bond can act as a nucleophile with the carbene in the formation of a cyclopropyl ring.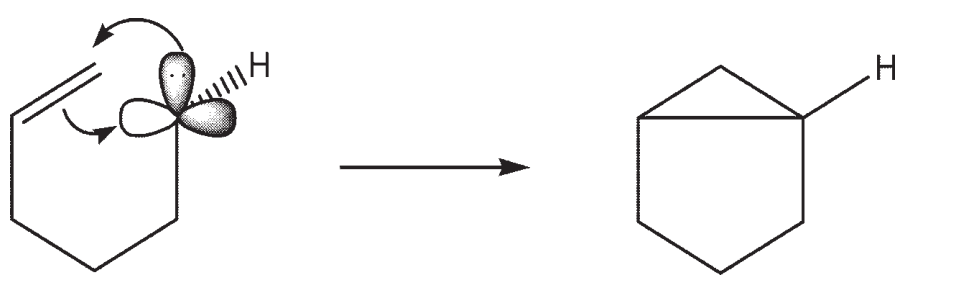
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q19: Which statement best summarizes the stereochemical outcomes
Q20: Which of the following structures have neighboring
Q21: Propose an explanation for the difference in
Q22: Explain why trans-1-chloro-2-phenylcyclohexane A would be expected
Q23: Structure A reacts faster with nucleophiles than
Q25: Explain why compound A below would be
Q26: Use the idea of neighboring group participation
Q27: Neighboring group effects are more strongly observed
Q28: How many different 13C NMR signals
Q29: Draw the mechanism of the following reaction;
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents