Consider the following representation of a voltaic cell. 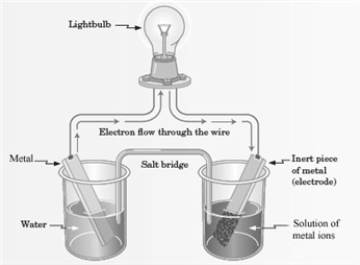 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
What is the identity of the "Metal" shown in the diagram?
A) Mg
B) Cu
C) Mg2+
D) Cu2+
Correct Answer:
Verified
Q127: How many calories are required to heat
Q128: The combustion of one mole of propane,
Q129: Assume each of the containers in the
Q130: Which of the following is true?
A) An
Q131: Consider the following representation of a voltaic
Q133: Examine the reaction taking place in the
Q134: Which of the following is true of
Q135: Examine the reaction taking place in the
Q136: The metabolism of one mole of glucose,
Q137: Consider the following representation of a voltaic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents