Consider the following representation of a voltaic cell. 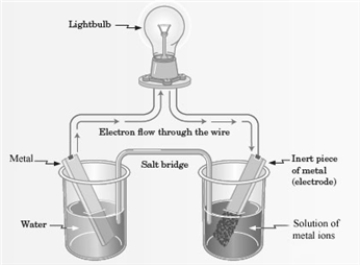 This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
This voltaic cell is represented by the following reaction: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
Which of the following is true of the Mg(s) shown in the reaction representing the voltaic cell?
A) It is the anode of the cell.
B) It is the place where reduction occurs.
C) It functions as the oxidizing agent.
D) All of these are correct.
Correct Answer:
Verified
Q128: The combustion of one mole of propane,
Q129: Assume each of the containers in the
Q130: Which of the following is true?
A) An
Q131: Consider the following representation of a voltaic
Q132: Consider the following representation of a voltaic
Q133: Examine the reaction taking place in the
Q134: Which of the following is true of
Q135: Examine the reaction taking place in the
Q136: The metabolism of one mole of glucose,
Q138: The image shows several types of burners
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents