Write the equilibrium equation that exists between calcium sulfate as a reactant and calcium oxide and sulfur trioxide as products.
A) 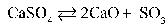
B) 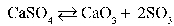
C) 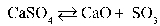
D) 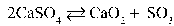
E) 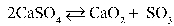
Refer to the reaction below for 28-29.
Given: 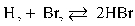
Correct Answer:
Verified
Q30: The equilibrium partial pressures of [H2O2] and
Q31: If [C2H6], [O2], [CO2], and Keq are
Q32: If the Kb for CN− is 1.6
Q33: If [OH−] is 2.02 × 10−7 M,
Q34: If [H2], [Cl2], and Keq are 0.315
Q36: Write the equilibrium equation that exists between
Q37: What is the KP expression for the
Q38: If the Ksp of SrSO4(s) is 3.8
Q39: What is the KP at 47.0°C for
Q40: The expression for the slight solubility of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents