What is the KP at 47.0°C for this reaction if the Keq is 2.36? 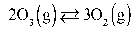 Assume R = 0.08205
Assume R = 0.08205 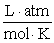
A) 31.2
B) 9.88
C) 0.0988
D) 62.0
E) 0.0889
Correct Answer:
Verified
Q34: If [H2], [Cl2], and Keq are 0.315
Q35: Write the equilibrium equation that exists between
Q36: Write the equilibrium equation that exists between
Q37: What is the KP expression for the
Q38: If the Ksp of SrSO4(s) is 3.8
Q40: The expression for the slight solubility of
Q41: What is the Keq at 7.00°C for
Q42: Which of the following will cause the
Q43: Which of the following will cause the
Q44: Which of the following is the right
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents