For the following reaction, the equilibrium constant 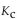 is 2.0 at a certain temperature. Bromine can be liquefied easily and removed from the reaction vessel as it is formed. If this is done, how will it affect the equilibrium reac
is 2.0 at a certain temperature. Bromine can be liquefied easily and removed from the reaction vessel as it is formed. If this is done, how will it affect the equilibrium reac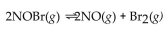
A) The equilibrium constant will change.
B) Less NO will be produced.
C) There will be a larger proportion NOBr in the vessel when equilibrium is reached.
D) The pressure in the vessel will increase.
E) More products will be produced as 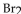 is removed.
is removed.
Correct Answer:
Verified
Q21: The reaction of hemoglobin with oxygen can
Q22: Q23: Q24: In the reaction of carbon dioxide with Q25: In an exothermic reaction, heat can be Q27: For the following reaction, the equilibrium constant Q28: When you open a bottle of a Q29: In the reaction of nitrogen gas with Q30: Carbon monoxide binds to hemoglobin 140 times Q31: Treatment of carbon monoxide poisoning can be![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents