The diagram shows a bag (with permeability characteristics similar to that of a normal cell) that contains a 100 mM solution of urea at time zero. The bag is placed in a beaker containing 100 mM glucose. Which of the following best describes the tonicity and osmolarity of the glucose solution as well as any changes in bag volume (assume that the bag volume is infinitely small compared to beaker volume)? 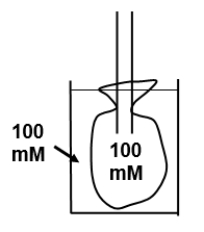
Correct Answer:
Verified
Q1: Human red blood cells (RBCs) and
Q2: The intracellular calcium ion concentration of ventricular
Q3: Which of the following transport mechanisms
Q4: A cell placed in a hypertonic solution
Q5: A cell is equilibrated in an aqueous
Q7: The diagram shows a model cell that
Q8: The diagram illustrates possible changes in red
Q9: The diagram illustrates possible changes in red
Q10: The diagram illustrates possible changes in red
Q11: Which of the following substances is most
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents