The Henderson-Hasselbalch equation is written as follows: 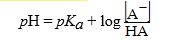
For what practical purpose is this equation used in the laboratory setting?
A) Calculating the optimum pH at which an enzymatic reaction occurs
B) Comparing the strength of an acid against a base
C) Determining the pH of a physiological reaction
D) Preparing a buffer at a specific pH
Correct Answer:
Verified
Q9: The computerization of modern laboratories has made
Q10: Which of the following is NOT a
Q11: What impact would an increase in the
Q12: Why is it more practical to measure
Q13: A laboratory technician measures the transmittance of
Q14: The Beer-Lambert Law (also called "Beer's Law")defines
Q15: Why is the kinetic assay considered a
Q16: You are asked to calculate the
Q18: The Friedewald equation is used to calculate
Q19: Which of the following best explains the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents