In the following galvanic cell: 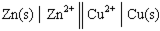 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:
A) spontaneous half of the reaction.
B) oxidation half-reaction.
C) anode of the cell.
D) reduction half-reaction.
Correct Answer:
Verified
Q21: The rusting of the sheet metal of
Q22: By convention, the standard hydrogen electrode (SHE)is
Q23: What is the cell potential (E0) f or
Q24: What is the cell potential (E
Q25: A salt bridge between half-reactions maintains the
Q27: Suppose that you have an iron rod
Q28: What is the cell potential (E
Q29: How many grams of silver are deposited
Q30: In the context of cell notations in
Q31: In a galvanic cell, oxidation occurs at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents