What is the cell potential (E 0 ) for a galvanic cell formed from the following two half- reactions? 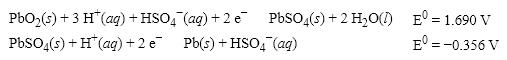
A) +1.334 V
B) − 2.046 V
C) +2.046 V
D) +2.758 V
Correct Answer:
Verified
Q23: What is the cell potential (E0) f or
Q24: What is the cell potential (E
Q25: A salt bridge between half-reactions maintains the
Q26: In the following galvanic cell: 
Q27: Suppose that you have an iron rod
Q29: How many grams of silver are deposited
Q30: In the context of cell notations in
Q31: In a galvanic cell, oxidation occurs at
Q32: The most prevalent type of primary battery
Q33: Identify the balanced chemical equation for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents