What is the cell potential (E0) f or a galvanic cell formed from the following two half-reactions? Assume that the cell temperature is 38°C and the operating pressure is 0.04 atm. 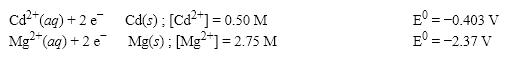
A) 1.90 V
B) 1.95 V
C) 1.99 V
D) 2.20 V
Correct Answer:
Verified
Q18: Electrolysis involves using an external electric current
Q19: The Nernst equation describes the pH required
Q20: Identify a true statement about a redox
Q21: The rusting of the sheet metal of
Q22: By convention, the standard hydrogen electrode (SHE)is
Q24: What is the cell potential (E
Q25: A salt bridge between half-reactions maintains the
Q26: In the following galvanic cell: 
Q27: Suppose that you have an iron rod
Q28: What is the cell potential (E
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents