When 10.0 g of ethane, C₂ H₆(g) , burns in oxygen, 519 kJ of heat are released. What is D H for the following thermochemical equation?
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O ( 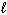 )
)
A) +3.12×103 kJ
B) +1.56×103 kJ
C) - 3.12×103 kJ
D) - 1.56×103 kJ
E) none of these
Correct Answer:
Verified
Q29: When a 50.0 g sample of a
Q30: How many calories of heat are required
Q31: When 5.00 g of butane, C4H10 (g),
Q32: Which statement below is true?
A) Some substances
Q33: How much heat must be removed from
Q35: How much heat must be added to
Q36: A chemical reaction releases enough heat to
Q37: The heat exchange of a certain exothermic
Q38: How much heat is required to raise
Q39: A reaction of 3.44 g of H2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents