When 5.00 g of butane, C4H10 (g) , burns in oxygen, 247 kJ of heat are released. What is D H for the following thermochemical equation?
2 C4H10 (g) + 13 O₂(g) 8 CO₂ (g) + 10 H₂ O ( 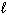 )
)
A) +2.87×103 kJ
B) +5.74×103 kJ
C) - 5.74×103 kJ
D) - 2.87×103 kJ
E) none of these
Correct Answer:
Verified
Q26: How much heat must be added to
Q27: When a 60.0 g sample of a
Q28: The reaction of 8.00 g of SO2
Q29: When a 50.0 g sample of a
Q30: How many calories of heat are required
Q32: Which statement below is true?
A) Some substances
Q33: How much heat must be removed from
Q34: When 10.0 g of ethane, C₂ H₆(g),
Q35: How much heat must be added to
Q36: A chemical reaction releases enough heat to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents